A Clinical-Stage Biotech Company
CellKure
Revolutionizing Cancer Treatment with Advanced T-Cell Immunotherapy

Transforming Cancer Care
At CellKure, we are pioneering the next evolution in T-cell therapy, harnessing the immune system to fight aggressive cancers. Our breakthrough platform provides a durable and scalable solution, starting with acute myeloid leukemia (AML) and expanding into solid tumors.
Scalable & Efficient Manufacturing
Simplified production for widespread accessibility
Multi-Antigen Targeting
Broad tumor recognition to reduce resistance
Natural T-Cell Activation
No genetic modifications required
Minimized Patient Burden
Designed for a streamlined treatment experience
Our Platform
Advancing T-Cell Therapy
CellKure’s technology represents a significant leap forward in cellular immunotherapy, improving effectiveness, safety, and scalability.
-
Key Advantages
- ✔ Multi-Antigen Targeting – Broad tumor recognition to reduce resistance
- ✔ Natural T-Cell Activation – No genetic modifications required
- ✔ Scalable & Efficient – Simplified production for widespread accessibility
- ✔ Minimized Patient Burden – Designed for a streamlined treatment experience
-
How It Works
- Our proprietary platform activates T-cells in a way that enhances their ability to detect and eliminate cancer, while maintaining long-term immune surveillance to help prevent relapse.

Our Platform
Advancing T-Cell Therapy
CellKure’s technology represents a significant leap forward in cellular immunotherapy, improving effectiveness, safety, and scalability.
-
Key Advantages
- ✔ Multi-Antigen Targeting – Broad tumor recognition to reduce resistance
- ✔ Natural T-Cell Activation – No genetic modifications required
- ✔ Scalable & Efficient – Simplified production for widespread accessibility
- ✔ Minimized Patient Burden – Designed for a streamlined treatment experience
-
How It Works
- Our proprietary platform activates T-cells in a way that enhances their ability to detect and eliminate cancer, while maintaining long-term immune surveillance to help prevent relapse.
How CellKure is Changing the Landscape
Our vision extends beyond AML, with our platform designed for future applications in solid tumors and autoimmune diseases.
Targeting High-Risk Patients
Addressing critical unmet needs.
Precision-Driven Approach
Enhancing natural immune responses.
Strategic Development
Advancing through clinical milestones toward regulatory approval.
Expanding Our Reach: Pipeline Overview
We are continuously advancing our programs, leveraging cutting-edge science and strategic collaborations to drive progress.
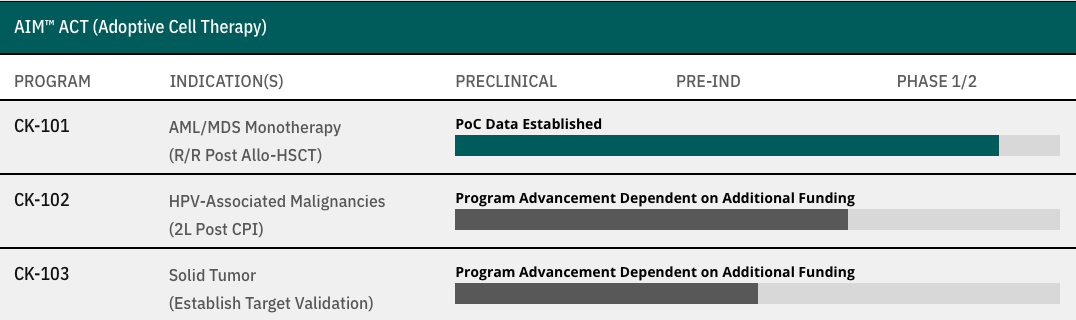
AIM™ ACT (Adoptive Cell Therapy)
PROGRAM
INDICATION(S)
PRECLINICAL
PRE-IND
PHASE 1/2
CK-101
AML/MDS Monotherapy
(R/R Post Allo-HSCT)
- PoC Data Established
CK-102
HPV-Associated Malignancies
(2L Post CPI)
- Program Advancement Dependent on Additional Funding
CK-103
Solid Tumor
(Establish Target Validation)
- Program Advancement Dependent on Additional Funding
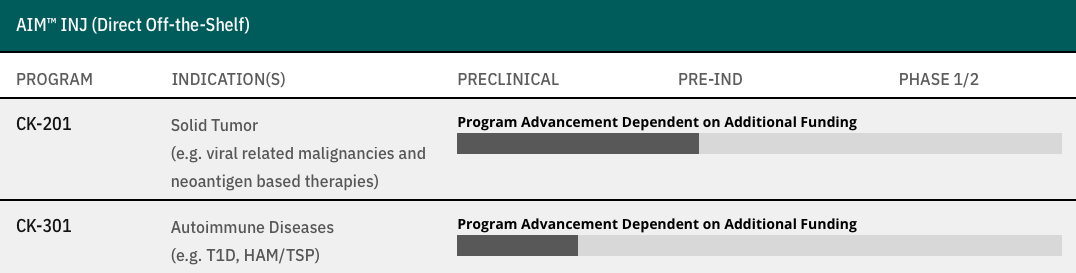
AIM™ INJ (Direct Off-the-Shelf)
PROGRAM
INDICATION(S)
PRECLINICAL
PRE-IND
PHASE 1/2
CK-201
Solid Tumor
(e.g. viral related malignancies and neoantigen based therapies)
- Program Advancement Dependent on Additional Funding
CK-301
Autoimmune Diseases
(e.g. T1D, HAM/TSP)
- Program Advancement Dependent on Additional Funding
How CellKure is Changing the Landscape
Our vision extends beyond AML, with our platform designed for future applications in solid tumors and autoimmune diseases.
Targeting High-Risk Patients
Addressing critical unmet needs.
Precision-Driven Approach
Enhancing natural immune responses.
Strategic Development
Advancing through clinical milestones toward regulatory approval.
Expanding Our Reach: Pipeline Overview
We are continuously advancing our programs, leveraging cutting-edge science and strategic collaborations to drive progress.
Why Partner with CellKure?
High-Growth Market Opportunity
The AML market is projected to surpass $5 billion by 2030, with strong demand for innovative therapies.
Scalable Pipeline for Multiple Cancers
CK-001 serves as the foundation for additional therapies targeting solid tumors, expanding our market reach.
De-Risked Clinical Development Pathway
Early proof-of-concept data, regulatory alignment, and a clear strategy for rapid clinical progression.
Strategic Positioning & Exit Potential
Strong potential for partnerships, IPO, or M&A, given recent billion-dollar deals in the cell therapy space.